COVIDRIVE´s second virtual site investigators meeting took place on December 14th, 2023. We welcomed participants from sites in Spain, Italy, […]
Author: COVIDRIVE consortium
Second annual face-to-face COVIDRIVE Steering Committee meeting
On November 28th and 29th, 2023, the members of the COVIDRIVE Steering Committee met in Valencia, Spain to discuss the […]
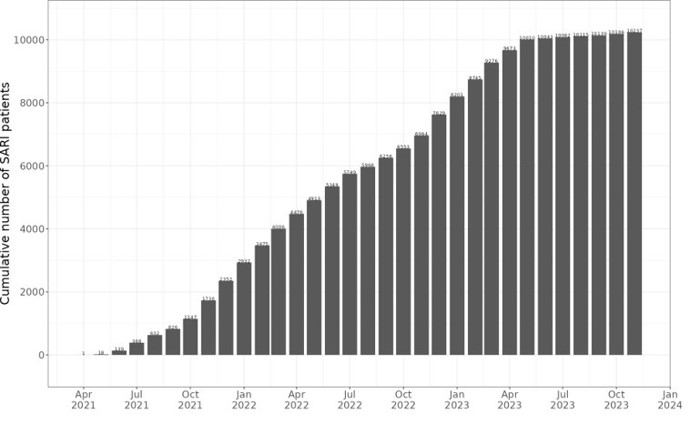
COVIDRIVE has reached a milestone: enrolment of over 10,000 patients
We are pleased to announce that the first COVIDRIVE study has recruited 10,038 patients across 17 hospitals in 5 European countries. This high recruitment number is important […]
AstraZeneca has published its first COVIDRIVE results in Lancet Regional Health Europe.
The study concluded that primary-series vaccination with AstraZeneca´s COVID-19 vaccine confers protection against COVID-19 hospitalisation with enduring levels of vaccine […]
Study investigator meeting in Copenhagen
On 16 April, 2023, during the European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) in Copenhagen, Denmark, a hybrid […]
First face-to-face COVIDRIVE Steering Committee meeting
On November 14th and 15th, 2022, the city of Valencia became the epicentre of COVIDRIVE Partnership discussions. The members of the COVIDRIVE Steering Committee (SC) connected to share the vaccine company-specific COVID-19 vaccine effectiveness interim results, to review all progress made and to brainstorm about vaccination preparedness and sustainability of the COVIDRIVE consortium. The group met at the Hotel Las Arenas and went through a packed two-day agenda.
New public-private partnership COVIDRIVE to assess brand-specific COVID-19 vaccine effectiveness in Europe
19/07/2021 – A new public-private partnership – COVIDRIVE – announced today that it will begin studies to assess the effectiveness of multiple COVID-19 vaccines in Europe to support the region’s public health response and to address the vaccine companies’ regulatory obligations. This multi-stakeholder partnership brings together public institutions, small medium enterprises and vaccine companies, including FISABIO (Spain), P95 (Belgium), THL (Finland), AstraZeneca (UK), CureVac (Germany), Janssen (Belgium), Sanofi-Pasteur (France) and GSK (Belgium).