WHAT IS A PUBLIC-PRIVATE PARTNERSHIP?
Public-Private Partnerships (PPPs) consist of joint initiatives between the private sector (i.e., pharmaceutical companies, small-medium enterprises…) and public sector (i.e., universities, public health institutes, research organizations) in which resources and responsibilities are shared in order to achieve a common goal. This cooperative arrangement follows tailored governance models, which clearly define the roles and responsibilities of the stakeholders, ensure complete transparency of their interactions and guarantee robustness and scientific value of the studies performed.
Benefits of Public–Private Partnership
Past and present health crises have highlighted that an efficient collaboration between the public and private sector is essential to address major health challenges. The main benefits of a PPP are the sharing of resources (e.g., economic funds, health infrastructure, knowledge and experience in disease and related vaccine and study designs, patients data and samples), risks, responsibilities, and rewards. PPPs foster collaboration and create multi-disciplinary networks of scientific experts.

id.DRIVE GOVERNANCE
id.DRIVE is a PPP funded by the pharmaceutical company partners leveraging health capacities from public partners and sites. It follows key guiding PPP principles collaboration aiming to:
- promote scientific exchanges between several stakeholders with various expertise
- optimally steer joint efforts towards the desired outcome within the time and resource constraints
- allow efficient decision-making, and guarantee scientific integrity of the study results with high quality standards.
id.DRIVE Governance structure is reflected in the following diagram:
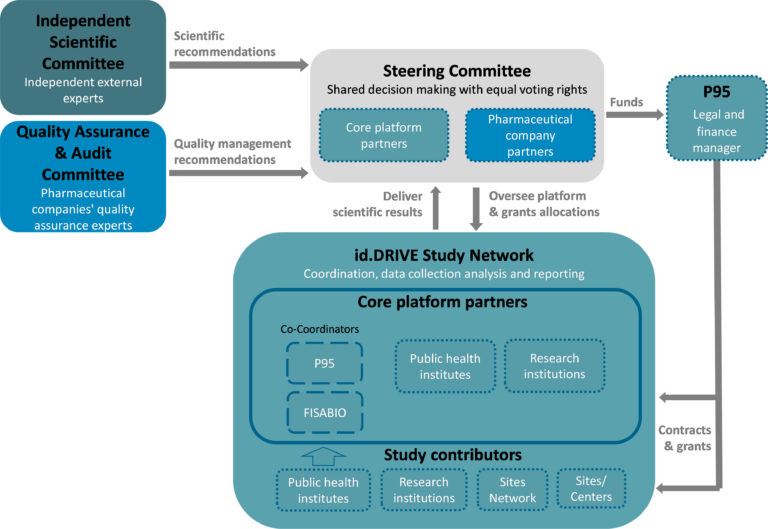
id.DRIVE is based on the following principles:
- The Steering committee decides on the study platform strategic direction, allocations of funds and resources for the studies.
The steering committee is composed of representatives from id.DRIVE partners. Decision authority is split equally between non-industry and pharmaceutical companies. - Master scientific and technical documents are developed by scientific experts from id.DRIVE partners.
- Scientific review of the master and study specific scientific documents is overseen by the Independent Scientific Committee (ISC) (see section below for ISC members).
- Quality control management and audit of the partnership and studies are overseen by the Quality assurance and audit committee (QAAC), composed of quality assurance experts from the pharmaceutical companies.
- At the study level, decision making and study conduct are the responsibilities of the corresponding Study Team (ST).
- Data collection is carried out at several independently operating Study Sites which constitute the study network. P95 is the sponsor of the studies. Study Sites remain owners of their data. Pharmaceutical companies’ partners are not permitted access to the data.
The id.DRIVE Governance Charter is available upon request, contact us at info@iddrive.eu
Independent Scientific Committee
To increase the robustness and transparency of the scientific results produced by the partnership, the scientific leadership of id.DRIVE is supported by an Independent Scientific Committee (ISC). The ISC’s mandate is to review and provide recommendations on the master and study-specific scientific documents.
The current members of id.DRIVE ISC are:
Dr Gabrielle Breugelmans


Prof. Elisabeth Miller
Prof. Harish Nair
Co-Director of the Centre for Global Health and Chair of Paediatrics Infectious Diseases at the University of Edinburgh. He currently leads or is the scientific coordinator of, the Preparing for RSV Immunisation and Surveillance in Europe (PROMISE), REspiratory Syncytial virus Consortium in EUrope (RESCEU), the Infectious Diseases Research Programme in the NIHR Global Health Research Unit on Respiratory Health (RESPIRE) and the Respiratory Virus Global Epidemiology Network (RSV GEN) which has developed the previous and current global paediatric lower respiratory infections morbidity and mortality estimates, specifically those relating to viral aetiologies (RSV, influenza, hMPV and hPIV). His current projects include work on child pneumonia, RSV, influenza and other infectious diseases like pneumococcus, meningococcus and Clostridium difficile. He is a co-founder of ReSViNET. He is a member of several WHO Technical Advisory Groups including the WHO SAGE working group on RSV and Chairs the External Advisory Group for WHO’s Global RSV Surveillance Programme. He is also a member of the international steering committee of the International RSV Society (within ISIRV).


Prof. Peter Smith
Professor of Tropical Epidemiology at the London School of Hygiene and Tropical Medicine (LSHTM). He joined LSHTM in 1979 to head the Medical Research Council Tropical Epidemiology Unit and then headed the Departments of Epidemiology and Population Sciences (1990-1996) and Infectious and Tropical Diseases (1997-2002). He has extensive experience in epidemiological and statistical research including large-scale intervention studies against tropical diseases, such as vaccine trials against malaria, dengue, Ebola Virus disease and more recently SARS-CoV-2. He currently serves on, or chairs, several international scientific advisory committees, including EDCTP and CEPI and the WHO SAGE Working groups on SARS-CoV-2 vaccines and on malaria vaccines.
Assoc. Prof. Sheena Sullivan
Infectious diseases epidemiologist, Adjunct Associate Professor in the School of Clinical Sciences, Monash University and former Head of Epidemiology for the WHO Collaborating Centre for Reference and Research on Influenza (WHOCC Influenza), Melbourne. Her work focuses on the valid estimation of vaccine effectiveness and understanding the immunological mechanisms underlying variations in observed effectiveness. She works with sentinel surveillance networks in Australia and real-world data studies in the USA to estimate the effectiveness and safety of influenza and COVID-19 vaccines. She collaborates on several sero-epidemiological studies to characterise immunological responses to influenza and SARS-CoV-2 infection and vaccination, with a specific focus on the effects of repeated influenza vaccination. She was on the WHO Strategic Advisory Group of Experts working group on influenza vaccines and has contributed to WHO guidance on the estimation of vaccine effectiveness. She has also been a technical advisor to the WHOon respiratory disease surveillance and using such data to estimate the burden of influenza.


Prof. Phillipe Beutels
Professor of Health Economics at University of Antwerp. He is the founder and director of the Centre for Health Economics Research & Modelling Infectious Diseases (CHERMID), and founder and co-director of the Interuniversity Simulation Models for Infectious Diseases (SIMID) consortium. He has been a frequent advisor for the WHO and member of pivotal WHO committees, such as the Immunization and Vaccines related Implementation Research Advisory Committee (IVIR-AC). He is internationally one of the most prolific and cited authors on health economic aspects of infectious diseases and vaccination. His main research interests are health economic evaluation, including quality of life and wellbeing research, modeling of infectious diseases, economics of infectious disease prevention, and preference elicitation for health care prioritization.
