STUDIES
id.DRIVE carries out different studies under a common data collection. Three studies are currently running: a respiratory viruses surveillance study, a COVID-19 Vaccine Effectiveness study (CVE), and a respiratory syncytial virus (RSV VE) Vaccine Effectiveness study.
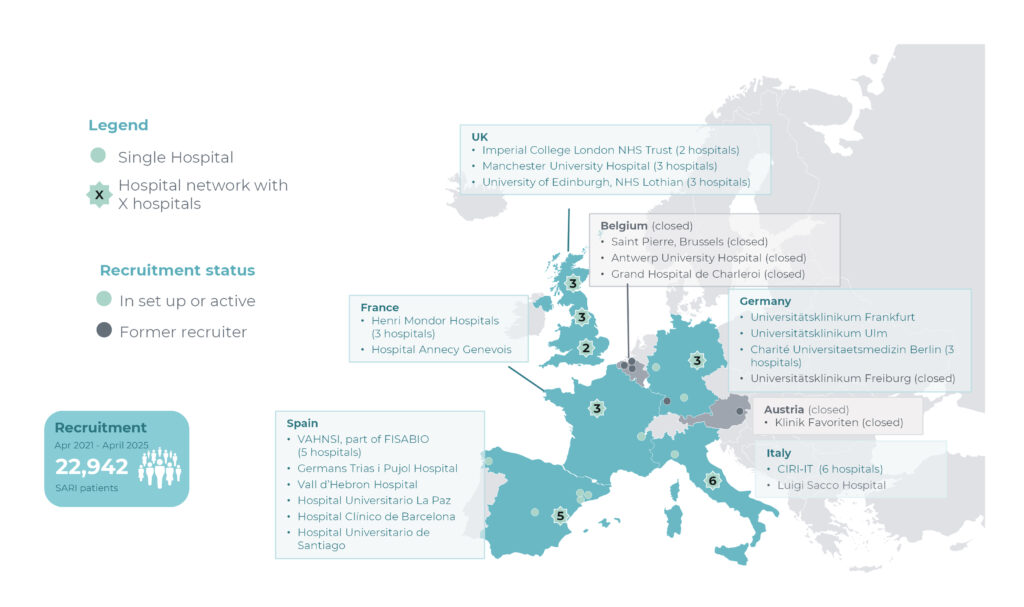
Patient recruitment started in September 2021 for the COVID-19 CVE study. The common data collection was launched on 26 July 2024 by including the surveillance study data, followed by RSV VE data collection in fall 2024. As of 16 April 2025, a total of 22,942 patients have been recruited across 26 hospitals in 6 European countries.
Study #1: COVID-19 Vaccine Effectiveness Study
This study uses a multi-country hospital-based test negative case control design. Patient recruitment was initiated in September 2021 and by 16 April 2025 22,942 patients have been recruited across 26 hospitals in 6 European countries.
The study was initiated at the request of AstraZeneca and Janssen in 2021 and was later joined by Novavax, Valneva and Pfizer to fulfil their regulatory commitments. The data collection is still ongoing.
🟦 Primary Objectives
- To estimate brand-specific CVE against hospitalisation due to laboratory-confirmed SARS-CoV-2 in SARI patients who have received the COVID-19 vaccine dose of interest, compared to selected comparator group.
🟨 Secondary Objectives
- To estimate brand-specific CVE against hospitalisation due to laboratory-confirmed SARS-CoV-2 in SARI patients who have received the COVID-19 vaccine dose of interest, compared to selected comparator group.
▪ by SARS-CoV-2 genetic variants.
▪ within populations of special interest (e.g., specific age groups, specific immunocompromised or chronic conditions, pregnant women).
▪ by time since last COVID-19 vaccine dose.
▪ by time between COVID-19 vaccine doses.
▪ by number or type(s) of the COVID-19 vaccine doses given prior to the last dose.
🟩 Exploratory Objectives
- To estimate brand-specific CVE against hospitalisation due to laboratory-confirmed SARS-CoV-2 in SARI patients who have received the COVID-19 vaccine dose of interest, compared to selected comparator group:
▪ by severity level;
▪ by calendar time;
▪ by history and calendar time of prior SARS-CoV-2 infection. - To estimate the brand-specific effect of COVID-19 vaccination in SARI patients who have received the COVID-19 vaccine dose of interest on length of hospital stay (in days) due to laboratory-confirmed SARS-CoV-2 admission, compared to selected comparator group.
The study is registered on the EMA Real-World Data Catalogue under “id.DRIVE (former COVIDRIVE) study of brand-specific COVID-19 vaccine effectiveness against severe COVID-19 disease in Europe” EUPAS42328.
The latest master protocol version can be found here.
Study #2: Surveillance of respiratory viruses study
This is a multi-country hospital-based study which monitors, per epidemiological year, viral respiratory infections in adults hospitalised for severe acute respiratory infection (SARI) and started 26 July 2024.
By 16 April 2025, 4,560 patients have been recruited across 20 hospitals in 4 European countries.
🟦 Primary Objectives
- To calculate the proportion of laboratory samples positive for each viral respiratory pathogen of interest, among adult patients hospitalised with SARI.
- To characterise adult SARI patients (including age, gender, protective- and risk factors, specific treatments, healthcare utilisation outcomes), overall and by viral respiratory pathogen of interest.
🟨 Secondary Objective
- To investigate the prevalence of co-infection with the viral respiratory pathogens of interest, in adult patients hospitalised with SARI, and the impact on healthcare utilisation outcomes.
🟩 Exploratory Objectives
- To calculate pathogen-specific SARI incidences and describe trends in adults (time period, country, level of severity).
- To perform feasibility assessments for future respiratory disease studies within the id.DRIVE Study Network (e.g., effectiveness studies, impact studies).
The study is registered on the EMA Real-World Data Catalogue under “id.DRIVE surveillance study of respiratory pathogens in adults hospitalised for severe acute respiratory infection (SARI) across Europe” EUPAS1000000012.
The latest protocol version can be found here.
Study #3: RSV Vaccine Effectiveness Study
This study uses a multi-country hospital-based test negative case control design. This study started in fall 2024.
By 16 April 2025, 72 patients have been recruited across 4 hospitals in 2 European countries.
🟦 Primary Objective
Objective 1
To estimate brand-specific respiratory syncytial virus (RSV) vaccine effectiveness (VE) against hospitalisation due to laboratory-confirmed RSV infection in modified severe acute respiratory infection (modified SARI)older adult patients.
As objective 1, but stratified by:
- RSV subtype A and B infection
- Population of special interest (e.g., age groups, gender)
- Time since vaccination
- Calendar time
- Modified SARI severity level
The study is registered on the EMA Real-World Data Catalogue under “id.DRIVE study of brand-specific respiratory syncytial virus (RSV) vaccine effectiveness in Europe” EUPAS1000000035.
The latest protocol version can be found here.
