STUDIES
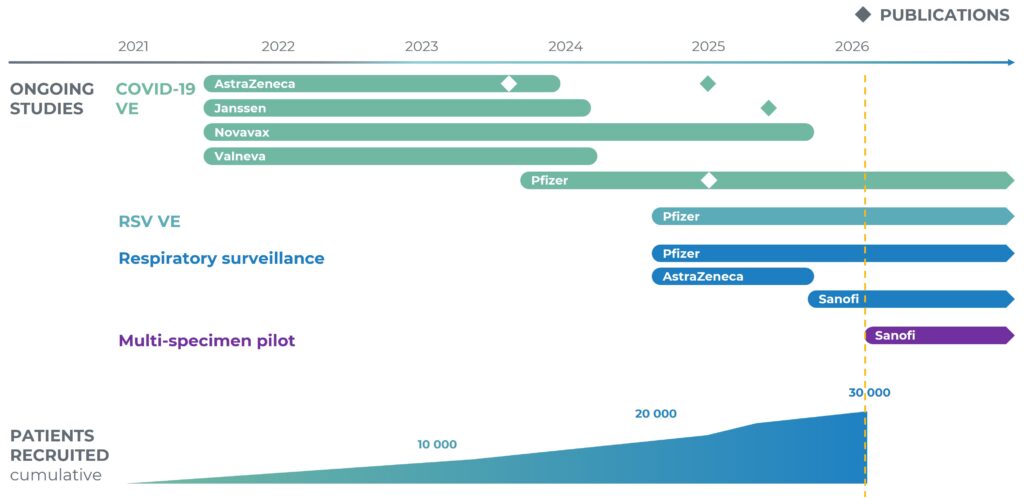
id.DRIVE carries out different studies under a common data collection. Three studies are currently running: a respiratory viruses surveillance study, a COVID-19 Vaccine Effectiveness study (CVE), and a respiratory syncytial virus (RSV VE) Vaccine Effectiveness study.
Patient recruitment started in September 2021 for the COVID-19 CVE study. The common data collection was launched on 26 July 2024 by including the surveillance study data, followed by RSV VE data collection in fall 2024, and the multi-specimen pilot study in early 2026. As of 11 of February 2026, a total of 33,041 patients have been recruited across 42 hospitals from 21 Study Contributors in 7 European countries.
Surveillance of respiratory viruses
- This yearly study started on 26 July 2024 and acts as an umbrella for the common data collection that feeds the VE studies.
- Multi-country hospital-based study monitoring viral respiratory infections in adults hospitalised for severe acute respiratory infection (SARI).
- The data collection for the 2025-26 epidemiological year runs from 1 September 2025 to 31 August 2026.
- By 11 of February 2026, 15 135 patients have been recruited in 33 hospitals across 15 Study Contributors in 5 European countries.
EMA Real-World Data Catalogue EUPAS1000000012
The latest protocol version can be found here.
- Calculate the proportion of positive laboratory samples for viral respiratory pathogens
- Characterise adult SARI patients
- Prevalence of co-infection and impact on healthcare utilisation outcomes
- Pathogen-specific SARI incidences
- Feasibility assessments for future respiratory disease studies
COVID-19 Vaccine Effectiveness
- The study was initiated at the request of AstraZeneca and Janssen in September 2021, and was joined later by Novavax, Valneva, and Pfizer to fulfil their regulatory commitments.
- Multi-country hospital-based test-negative case-control design.
- The data collection has been extended for the 2025-26 season.
- By 11 of February 2026, 32 594 patients have been recruited across 39 hospitals in 7 European countries.
EMA Real-World Data Catalogue EUPAS42328.
The latest master protocol version can be found here.
- Brand-specific COVID-19 vaccine effectiveness
- by SARS-CoV-2 genetic variants
- within populations of special interest
- by time since last dose
- by time between doses
- by number or type of doses given prior to last dose
- Brand-specific COVID-19 vaccine effectiveness:
- by severity level
- by calendar time
- by history and calendar time of prior infection
- Effect of vaccination on length of hospital stay
RSV Vaccine Effectiveness
- The study was initiated at the request of Pfizer in fall 2024.
- Multi-country hospital-based test-negative case-control design.
- The data collection has been extended for the 2025-26 season.
- By 11 of February 2026, 1 134 patients have been recruited across 13 hospitals in 3 European countries.
EMA Real-World Data Catalogue EUPAS1000000035.
The latest protocol version can be found here.
- Brand-specific RSV vaccine effectiveness
- by RSV subtype A and B infection
- by population of special interest
- by time since vaccination
- by calendar time
- by modified SARI severity level
Multi-specimen pilot study
- Pilot study to assess the detection of RSV, human metapneumovirus (hMPV), and parainfluenza virus (PIV) when using multi-specimen collection compared to a single nasopharyngeal swab.
- Aim to gain insights into the possible underestimation of RSV, hMPV, and PIV detection rates in patients with an (severe) acute respiratory infection.
- Prospective cohort pilot study conducted in a network of healthcare settings in Valencia (Spain).
- This pilot study targets the enrolment of approximately 1000 (s)ARI patients.
- As of 13 February 2026, 299 (s)ARI patients have been enrolled (of which 153 in the hospital setting).
- Data collection running from 7 January 2026 until 31 July 2026.
- Assess detection rates of RSV, hMPV and PIV in adult (s)ARI patients
- Assess detection rates of RSV, hMPV and PIV:
- by age categories, sex at birth, time since symptom onset categories, setting, diagnosis and common chronic conditions
- Assess RSV detection rates by RSV vaccination status
- Assess SARS-CoV-2 detection rates by SARS-CoV-2 vaccination status
- Assess Influenza detection rates by Influenza vaccination status
- Assess detection rates of co-infections
- Assess detection rates of RSV, hMPV and PIV by subtype or serotype
- Assess detection rates of viral pathogens tested by multiplex panel RT-PCR